Coating a copper sample by Ni-Sic composite coating and studying its corrosion behavior
Keywords:
Copper sample , Composite coating , Corrosion behaviour , Substrate Materials , Nickel sulfateAbstract
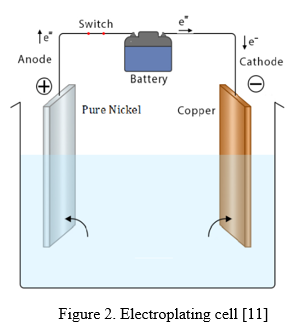
The electroplating of Ni-SiC composite coatings was conducted by submerging a brass substrate in a nickel sulfate solution with a SiC suspension. The composite coatings and brass substrate were evaluated for corrosion behaviour by generating Tafel curves in a 3.5% NaCl solution at room temperature. The composite electrolyte exhibited a greater cathodic polarization potential compared to the Cu substrate. The wear characteristics of the Ni coating, Cu substrate, and Ni-SiC composite coating made with an HT-8360 rotary disk were investigated in a study. Furthermore, the SiC nanoparticles co-deposited with Ni showed a homogeneous distribution within the Ni-SiC matrix. Consequently, there was a noticeable improvement in the Ni-SiC composite coating's microhardness and wear resistance.
AT The salt spray experiments were performed in a salt spray fog chamber, following the specifications outlined in ASTM-B-117. Changes in adhesion and blister formation, as well as those associated with corrosion, were examined.
Photographs of the specimens, which had been coated with a protective layer, were taken after the salt spray test. The percentage is a vital indication that precisely reflects the degree of corrosion resistance demonstrated by a coated specimen. There is no indication of corrosion.
Downloads
References
Nayak, B., Anwar, S., & Anwar, S. (2024). Mechanical and corrosion resistance behavior of ultrasonication-assisted electrodeposited Ni–SiC nanocomposite coatings. Physica B: Condensed Matter, 675, 415585.?
Jarz?bek, D. M., Dzieko?ski, C., Dera, W., Chrzanowska, J., & Wojciechowski, T. (2018). Influence of Cu coating of SiC particles on mechanical properties of Ni/SiC co-electrodeposited composites. Ceramics International, 44(17), 21750-21758.?
Calderón, J. A., Henao, J. E., & Gómez, M. A. (2014). Erosion–corrosion resistance of Ni composite coatings with embedded SiC nanoparticles. Electrochimica Acta, 124, 190-198.?
Ramezani, M., Mohd Ripin, Z., Pasang, T., & Jiang, C. P. (2023). Surface engineering of metals: techniques, characterizations and applications. Metals, 13(7), 1299.?
Rahmani, H., Aliofkhazraei, M., & Karimzadeh, A. (2017). Corrosion and wear properties of electrodeposited tertiary nanocomposite Zn–Ni (Alumina–Yittria–Geraphene) coating. Surface Review and Letters, 24(05), 1750066.?
Aliofkhazraei, M., Walsh, F. C., Zangari, G., Köçkar, H., Alper, M., Rizal, C., ... & Allahyarzadeh, M. H. (2021). Development of electrodeposited multilayer coatings: A review of fabrication, microstructure, properties and applications. Applied Surface Science Advances, 6, 100141.?
Schalk, N., Tkadletz, M., & Mitterer, C. (2022). Hard coatings for cutting applications: Physical vs. chemical vapor deposition and future challenges for the community. Surface and Coatings Technology, 429, 127949.?
Rajan, T. V., Sharma, C. P., & Sharma, A. (2023). Heat treatment: principles and techniques. PHI Learning Pvt. Ltd.
Nayak, B., Anwar, S., & Anwar, S. (2024). Mechanical and corrosion resistance behavior of ultrasonication-assisted electrodeposited Ni–SiC nanocomposite coatings. Physica B: Condensed Matter, 675, 415585.?
Zhang, X., Shao, Y., Sun, K., Fan, M., Zhang, S., & Hu, X. (2023). Introduction of NiSO4 to Ni/SiO2 catalyst in hydrogenation of furfuryl alcohol: Tailoring metallic nickel sites to switch major product from tetrahydrofurfuryl alcohol to cyclopentanone. Molecular Catalysis, 542, 113136.?
Fadiel, A. (2023). Electroplating Of Ni-Co Alloy In A Sodium Citrate Bath On Brass And Studying Some Effects On The Layer Thickness And Hardness.?
Leiden, A., Kölle, S., Thiede, S., Schmid, K., Metzner, M., & Herrmann, C. (2020). Model-based analysis, control and dosing of electroplating electrolytes. The International Journal of Advanced Manufacturing Technology, 111(5), 1751-1766.?
Leiden, A., Kölle, S., Thiede, S., Schmid, K., Metzner, M., & Herrmann, C. (2020). Model-based analysis, control and dosing of electroplating electrolytes. The International Journal of Advanced Manufacturing Technology, 111(5), 1751-1766.?
Banthia, S., Sengupta, S., Das, S., & Das, K. (2019). Synthesis and characterization of novel Cu, Cu-SiC functionally graded coating by pulse reverse electrodeposition. Applied Surface Science, 467, 567-579.?
Kauranen, K. (2024). Corrosion protection improvement validation with the use of salt spray testing.?
ASTM Committee G-1 on Corrosion of Metals. (2011). Standard Practice for Operating Salt Spray (fog) Apparatus. ASTM International.?
Zhou, Y., Xie, F. Q., Wu, X. Q., Zhao, W. D., & Chen, X. (2017). A novel plating apparatus for electrodeposition of Ni-SiC composite coatings using circulating-solution co-deposition technique. Journal of Alloys and Compounds, 699, 366-377.?
Tkadletz, M., Schalk, N., Daniel, R., Keckes, J., Czettl, C., & Mitterer, C. (2016). Advanced characterization methods for wear resistant hard coatings: a review on recent progress. Surface and Coatings Technology, 285, 31-46.?
Almabrouk, A. M., Khalid, H. M., & Fadiel, A. F. A. Study of Corrosion Behaviour Using Tafel Diagrams for a Brass Plate Electroplated with an N-Co Alloy.?
Ilmal, Y. R., Preston, S. J., Endri, P. Y., Bobby, D., Mula, T., Latif, A. M. Z., & Hari, P. B. (2022). Corrosion of Brass Fishing Vessel Propeller in Artificial Seawater. Majalah Ilmiah Pengkajian Industri, 16(1).?
Yaqin, R. I., Siahaan, J. P., Priharanto, Y. E., Demeianto, B., Tumpu, M., Abrori, M. Z. L., & Priyambodo, B. H. (2022). Corrosion of Brass Fishing Vessel Propeller in Artificial Seawater. Majalah Ilmiah Pengkajian Industri; Journal of Industrial Research and Innovation, 16(1), 11-17.?
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2025 Yoonis A.M. Esham, Ali F .Ali FADIEL, Moktar A. Hashem Mohamad, Hafiez M.B. Khalid

This work is licensed under a Creative Commons Attribution 4.0 International License.
Copyright on any article in the International Journal of Engineering and Applied Physics is retained by the author(s) under the Creative Commons license, which permits unrestricted use, distribution, and reproduction provided the original work is properly cited.
License agreement
Authors grant IJEAP a license to publish the article and identify IJEAP as the original publisher.
Authors also grant any third party the right to use, distribute and reproduce the article in any medium, provided the original work is properly cited.














