Structural and Electronic Properties of Gallium Phosphate Semiconductor in Wurtzite Rock-Salt and Zinc-Blende
Keywords:
Density functional theory, Gallium Phosphate, Band gap and Semiconductor, Pseudo-Potential, SemiconductorAbstract
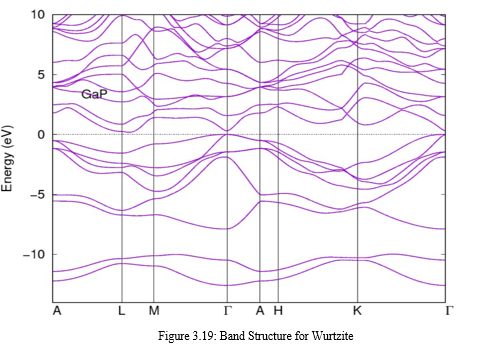
First principle method was used to study the structural and electronic properties of Gallium Phosphate in Wurtzite, zinc blende and rock salt using the molecular dynamics density functional theory implemented on the Quantum Espresso software. For this work the choice of the pseudo-potential is the GGA, scf calculation was used for zinc blende and rock salt to test the convergence of kinetic energy cut-off, lattice parameter, number of k-point with respect to 1mRy energy and 0.5kbar pressure. The same was repeated in wurtzite but vc-relax was used, all the plots obtained for the three structure converges towards the set threshold, the plot of band structure and density of state reveals that rock salt is a metal due to absence of band gap while other are semi-conductors with an indirect band gap, a plot of energy versus enthalpy shows that there’s a transition from zinc blende to rock salt with about 18.73% decrease in volume from zinc blende to rock salt and this occur at a pressure of 29.07GPa, it is noteworthy that Gallium Phosphate was found to be more stable in zinc blende.
Downloads
References
R.W.G. Wyckoff, Crystal Structures, second ed., Krieger, Malabar, (1986).
O. Madelung, Semiconductors: Data Handbook, Springer, Berlin, (2004).
O. Madelung, Landolt-Bornstein, Numerical Data and Functional Relationships in Science and Technology New Series, Springer, Berlin, (1982).
E. Schroten, A. Geossens, J. Schoonman, J. Appl. Phys. 83 (3) (1998) 1660 (references given therein).
B. Bouhafs, H. Aourag, M. Cartier, J. Phys.: Condens. Matter 12, 5655 (2000)
R.M. Wentzcovitch, M.L. Cohen, J. Phys. C: Solid State Phys. 19, 6791 (1986).
L. Lin, G.T. Woods, T.A. Callcott, Phys. Rev. B 63, 235107 (2001).
M. Ferhat, A. Zaoui, M. Certier, H. Aourag, Physica B 252, 229 (1998)
P. Hohenberg, W. Kohn, Phys. Rev. 136, 864 (1964).
W. Kohn, L.J. Sham, Phys. Rev. A 140, 1133 (1965).
W.E. Pickett, Comput. Phys. Rep. 9, 117 (1989).
J.P. Perdew, Y. Wang, Phys. Rev. B 45, 13244 (1992).
J.P. Perdew, K. Burke, M. Emzerhof, Phys. Rev. Lett. 77, 3865 (1996).
E. Engel, S.H. Vosko, Phys. Rev. B 47, 13164 (1993).
Y. Juan, E. Kaxiras Phys. Rev. B, 48, 14944, (1993).
P. D. Ye, J. Vac. Sci. Technol. A, 26, 696, (2008).
J. Phys.: Condens. Matter 14, 9579–9587 (2002).
H.J. Kim, B.J. Kim, T.W. Kim, K.H. Yoo, Appl. Surf. Sci. 255, 5048 (2009).
S. Fridjine, S. Touihri, K. Boubaker, M. Amlouk, J. Cryst. Growth 312, 202 (2010).
S. Park, H. Kim, C. Jin, C. Lee, Curr. Appl. Phys. 12, 499 (2012).
K.M.M. Abo-Hassan, M.R. Muhamad, S. Radhakrishna, Physica B 358, 256 (2005).
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2022 Solomon Tahiru Tonga, Yohanna Jacob Baro

This work is licensed under a Creative Commons Attribution 4.0 International License.
Copyright on any article in the International Journal of Engineering and Applied Physics is retained by the author(s) under the Creative Commons license, which permits unrestricted use, distribution, and reproduction provided the original work is properly cited.
License agreement
Authors grant IJEAP a license to publish the article and identify IJEAP as the original publisher.
Authors also grant any third party the right to use, distribute and reproduce the article in any medium, provided the original work is properly cited.
Most read articles by the same author(s)
- Solomon Tahiru Tonga, T. Tiye, Electronic and Structural Properties of an Undoped Sodium Iodide , International Journal of Engineering and Applied Physics: Vol. 4 No. 2: May 2024














