Substituent analysis for accurate prediction of the acidity constants of phosphonic acids
Keywords:
Substituent effects, Linear regression, Acidity constants, Phosphonic acids, Statistical analysisAbstract
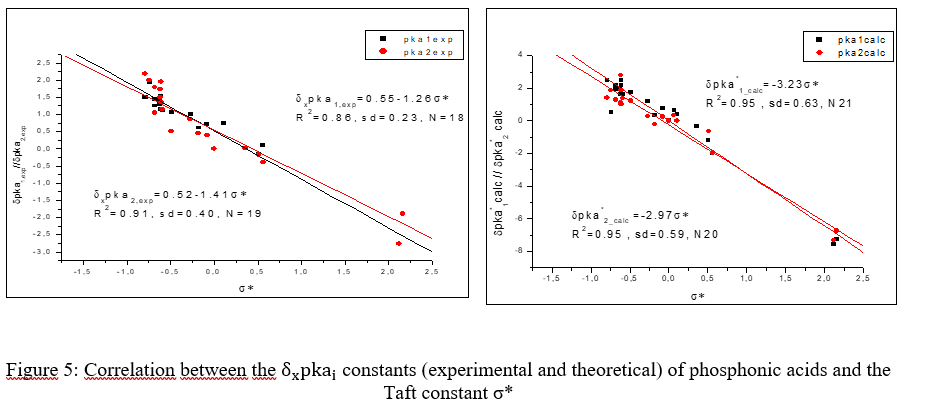
This study aims to develop simple and reliable models to predict the pKa of phosphonic acids using empirical substituent effects descriptors. The effects of substituents on the acidity and basicity of organic acids have been examined using various approaches, such as the Taft-Topsom model. This study used linear regression methods to construct equations that predict the acidity constants of phosphonic acids. The validity of the linear regressions was confirmed through statistical analysis of parameters and residuals. However, it is essential to note that the models are limited to substituents with tabulated Taft parameters. Despite these limitations, the developed models provide a simple and effective method for estimating the acidity constants of phosphonic acids with a confidence interval greater than 95%. This study aims to analyze the effect of substituents on the dissociation of phosphonic acid and propose prediction models for new phosphonic derivatives
Downloads
References
R. Bouaddi, L. El Maimouni, and A. El Hammadi, “A theoretical calculation of relative pKas of phosphonic acids in an aqueous solution using idodesmic reaction and the Polarisable Continuum Model,” Int. J. Comput. Eng. Res., vol. 9, no. 2, 2019.
M. Lew, C. Bagw, L. K. E. Hardebeck, and S. Wireduaah, “8-340-1-Pb,” no. April, 2012.
W. P. Ozimi?ski and J. C. Dobrowolski, “?- and ?-electron contributions to the substituent effect: Natural population analysis,” J. Phys. Org. Chem., vol. 22, no. 8, pp. 769–778, 2009, doi: 10.1002/poc.1530.
H. C. Shands, “Compte rendu,” Semiotica, vol. 3, no. 3, pp. 280–287, 1971, doi: 10.1515/semi.1971.3.3.280.
B. Cl, “Table A.l comprises an extensive compilation of Hammett.”
S. Rachuru, A. A. Skelton, and J. Vandanapu, “Application of Hammett equation to hydrogen bond interactions of benzoic acid in chloroform/water system and explanation for non-linear Hammett relation to partition coefficients for the same system,” Comput. Theor. Chem., vol. 1190, no. August, p. 113024, 2020, doi: 10.1016/j.comptc.2020.113024.
A. Campero and J. A. Diáz Ponce, “Averaged Scale in Electronegativity Joined to Physicochemical Perturbations. Consequences of Periodicity,” ACS Omega, vol. 5, no. 40, pp. 25520–25542, 2020, doi: 10.1021/acsomega.0c00256.
Y. Xing, J. Ku, W. Fu, L. Wang, and H. Chen, “Inductive effect between atomically dispersed iridium and transition-metal hydroxide nanosheets enables highly efficient oxygen evolution reaction,” Chem. Eng. J., vol. 395, no. January, p. 125149, 2020, doi: 10.1016/j.cej.2020.125149.
M. Manassir and A. H. Pakiari, “An electronic properties investigation to interpret the substituent constants of monosubstituted benzene derivatives,” J. Mol. Graph. Model., vol. 92, pp. 201–207, 2019, doi: 10.1016/j.jmgm.2019.07.017.
B. ying Wei, C. tun Cao, and C. zhong Cao, “Influences of polarizability effect of alkyl group and homoring competition effect of substituents on the fluorescence emission spectra of salen-type Schiff bases,” J. Phys. Org. Chem., vol. 34, no. 11, pp. 1–8, 2021, doi: 10.1002/poc.4263.
S. Herbers, P. Buschmann, J. Wang, K. G. Lengsfeld, K. P. R. Nair, and J. U. Grabow, “Reactivity and rotational spectra: The old concept of substitution effects,” Phys. Chem. Chem. Phys., vol. 22, no. 20, pp. 11490–11497, 2020, doi: 10.1039/d0cp01145b.
B. A. Saleh, S. A. Al-Shawi, and G. F. Fadhil, “Substituent Effect Study for 13C? SCS in a Meta-Styrene Series. Reynolds Dual Substituent Parameter Model Investigation,” J. Phys. Chem. A, vol. 125, no. 36, pp. 7759–7768, 2021, doi: 10.1021/acs.jpca.1c02645.
M. G. Zahl, R. Fossheim, K. J. Børve, L. J. Sæthre, and T. D. Thomas, “Electronic Properties of Chlorine, Methyl, and Chloromethyl as Substituents to the Ethylene Group-Viewed from the Core of Carbon,” J. Phys. Chem. A, vol. 119, no. 36, pp. 9481–9493, 2015, doi: 10.1021/acs.jpca.5b05494.
C. A. Jinfeng, R. D. Topsom, U. S. A. Ca, and A. G. Best, “ACIDITIES OF SUBSTITUTED,” vol. 168, pp. 141–146, 1988.
J. Palomar and J. L. G. De Paz, “Protonation study of some enamine systems,” vol. 541, pp. 111–117, 2001.
F. The, “AMERICAN CHEMICAL,” vol. 112, no. 6, pp. 2047–2052, 1990.
P. Taylor et al., “Critical Reviews in Analytical Chemistry The Correlation Coefficient?: An Overview The Correlation Coefficient?: An Overview,” no. September 2013, pp. 37–41, 2007, doi: 10.1080/10408340500526766.
L. D. Freedliaru, A. S. D. G. Dom, and N. Carolina, “ACIDS,” 1956.
E. Bosch, K. H. A. H. A.- Y, and K. B. S.-B. Oh-, “IONIC EQUILIBRIA IN NEUTRAL AMPHIPROTIC SOLVENTS?; RESOLUTION OF ACID STRENGTH IN tert -BUTY L ALCOHOL [ H + l [ A-I P + I @ -1 + W-1 ),” vol. 36, no. 6, 1989.
“The Acid Dissociation Constants of Aromatic Phosphonic Acids. Ortho Substituents,” vol. 540, no. 11, pp. 257–258, 1946.
Z. Jinhua, T. Kleinöder, and J. Gasteiger, “Prediction of pKa values for aliphatic carboxylic acids and alcohols with empirical atomic charge descriptors,” J. Chem. Inf. Model., vol. 46, no. 6, pp. 2256–2266, 2006, doi: 10.1021/ci060129d.
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2023 rachid bouaddi

This work is licensed under a Creative Commons Attribution 4.0 International License.
Copyright on any article in the International Journal of Engineering and Applied Physics is retained by the author(s) under the Creative Commons license, which permits unrestricted use, distribution, and reproduction provided the original work is properly cited.
License agreement
Authors grant IJEAP a license to publish the article and identify IJEAP as the original publisher.
Authors also grant any third party the right to use, distribute and reproduce the article in any medium, provided the original work is properly cited.














